Extracellular potentials and ECGs
See code in GitLab.
Author: Gernot Plank gernot.plank@medunigraz.at, Fernando Campos fernando.campos@kcl.ac.uk
Overview
This example explains the background of computing extracellular potentials and electrocardiograms (ECGs) using different techniques. The techniques used have their specific advantages depending on the application scenario. In this example the focus is on using \(\phi_{\rm e}\) to compute unipolar extracellular electrograms and compare these with fullblown bidomain simulations.
- Recovery of extracellular potentials: Recovery techniques are efficient when potentials shall be computed for a limited number of field points. An important limitation is the underlying assumption that cardiac electric sources are immersed in an unbounded conductive medium of conductivity \(\sigma_\mathrm{b}\). The technique is not overly efficient for a large number of field points such as in a body surface potential mapping application. There BEM-based or lead field approaches are better suitable.
- bidomain: When using a bidomain formulation, extracellular current flow and potentials are computed everywhere in the entire domain. This approach is most accurate and considered a gold standard for ECG modeling. However, the main disadvantage is the significantly increased computational cost. First, the entire domain must be explicitly discretized, that is, the tissue domain plus a bath or torso domain where extracellular potentials can be recorded. Secondly, bidomain requires the solution of an elliptic PDE which tends to be more costly than the plain reaction-diffusion monodomain equation, roughly by one order of magnitude.
- pseudo bidomain: In this case very similar considerations hold as in the bidomain case. The main advantage of a pseudo-bidomain approach as published by Bishop & Plank are the significant savings in computational cost. A pseudo-bidomain is only marginally more expensive than a monodomain simulation as extracellular potentials are only solved at output granularity. The main disadvantage of the pseudo-bidomain approach is that any feedback of current flow in the extracellular medium upon excitation spread remains unaccounted for.
Theoretical background and more rigorous validation of the methods are found in the electrocardiogram section of the manual and in 1 and 2 .
To run the experiments of this example, change directories as follows:
cd ${EXAMPLES}/02_EP_tissue/07_extracellularObjectives
This example aims to achieve the following objectives:
- Understand how to use the openCARP \(\phi_{\rm e}\)-recovery feature
- Analyse differences between \(\phi_{\rm e}\)-recovery and full bidomain-based extracellular potentials
- Examine the effect of bath size upon extracellular potentials in the bath medium and its relationship to recovered potentials.
Setup
A simple reduced wedge setup is used for this example.
While a reasonable transmural width of 10 mm is used, in the circumferential direction
the spatial extent is limited to the used spatial resolution for the sake of computational efficiency.
That is, the wedge is a 3D object, but comprises only one layer of elements
along the circumferential direction (x-axis), rendering the setup almost 2D.
The wedge contains transmural as well as apico-basal heterogeneities
which are based on the study of Boukens et al
3
.
The wedge uses standard transmural fiber rotation.
Stimulation sites to initiate propagation can be chosen at six different locations
along the endocardial and epicardial surface.
The size of the bath is exposed as an input parameter and can be varied.
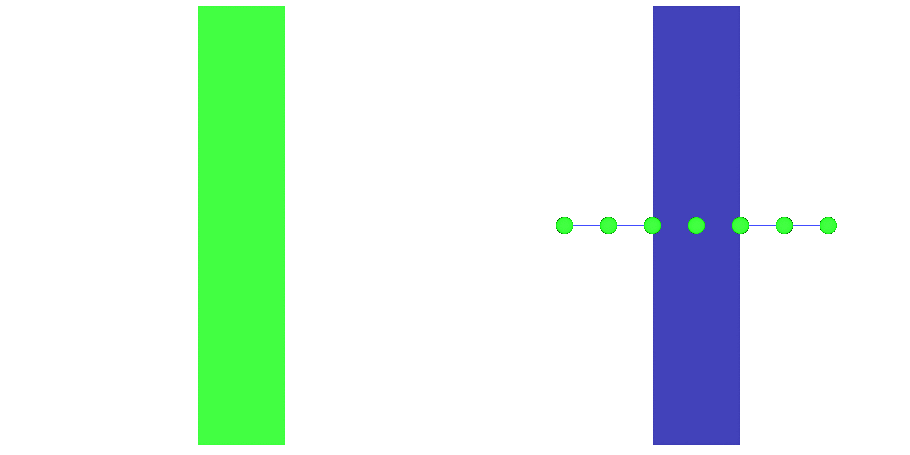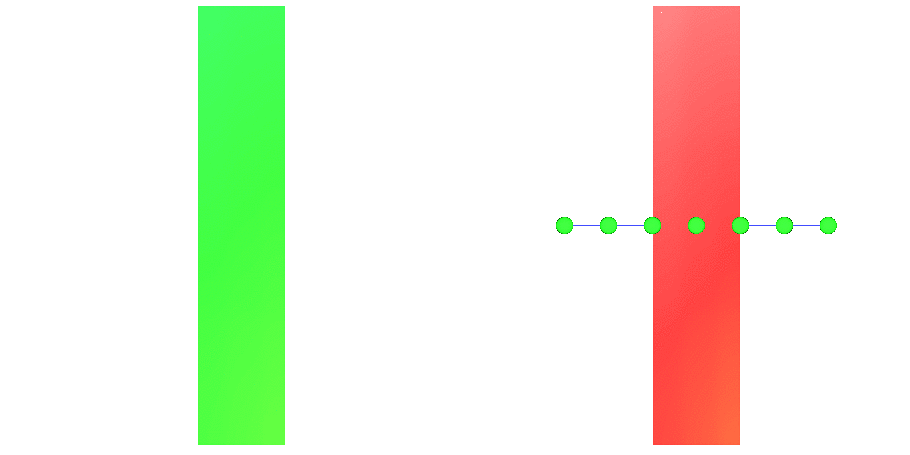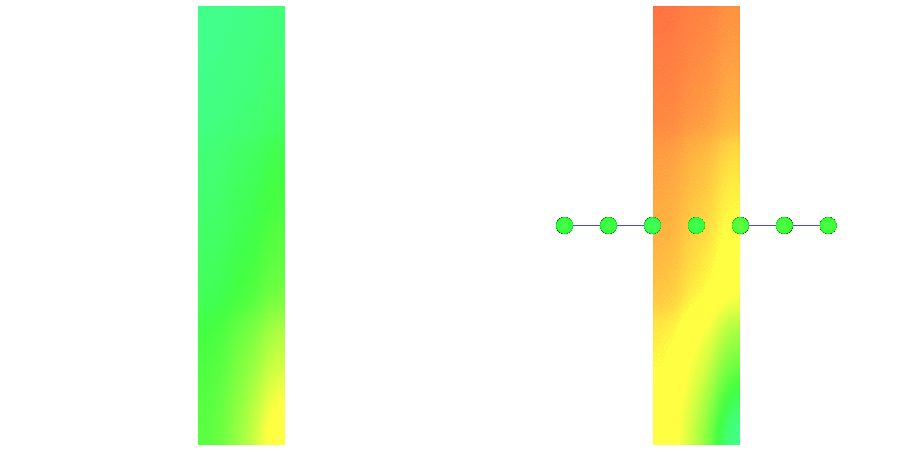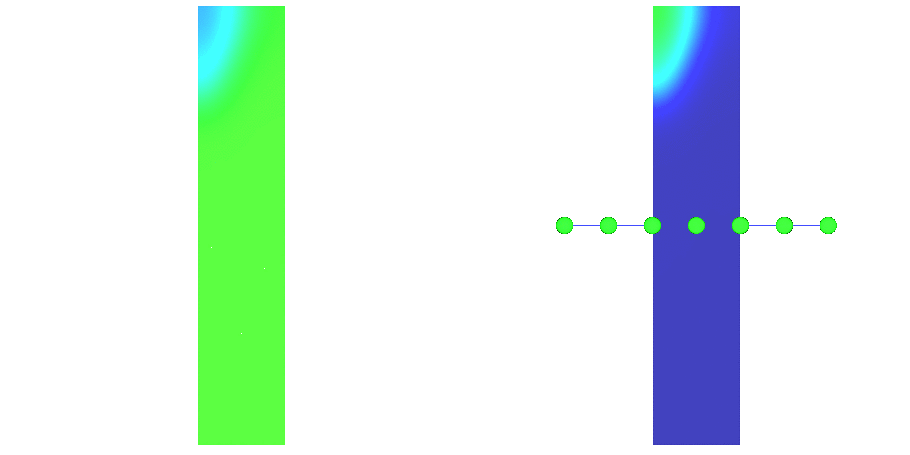
An overview of the experimental setup is given in fig-tutorial-ecg A).
Default visualization outputs showing local activation time, action potential propagation
and extracellular potentials are illustrated in fig-tutorial-ecg B).
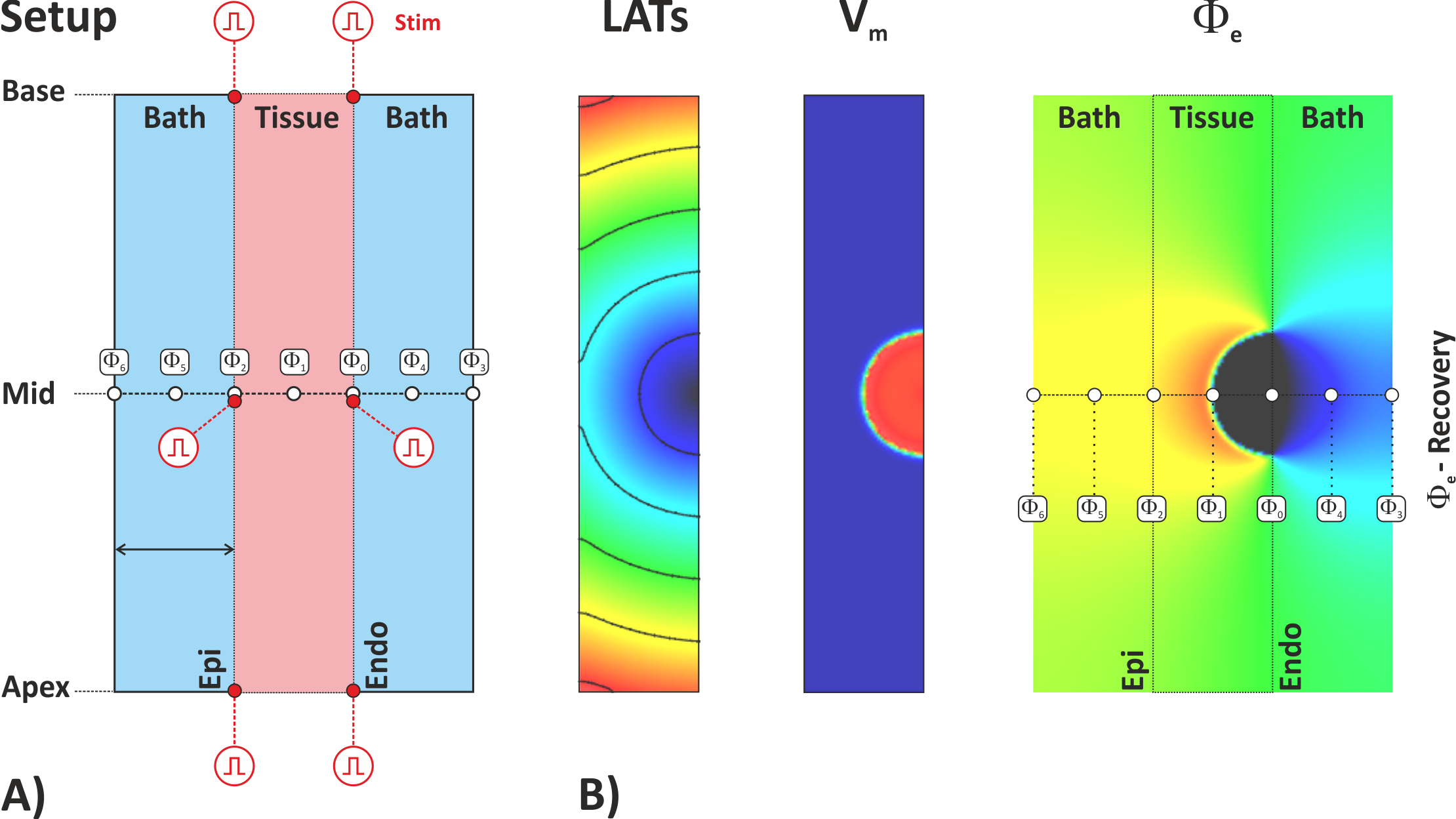
bath>0) extra recovery sites are located
at the surface of the bath and in the center of the bath for both bath compartments
interfacing with endocardial and epicardial surfaces.Input Parametes
The following input parameters are exposed to steer the experiment:
--duration DURATION
Duration of simulation [ms].
Choosing a value of around 70 ms covers activation only
and thus yields only the depolarization complex of the ECG.
A value of 350 ms covers also repolarization and T-waves.
--resolution RESOLUTION
choose mesh resolution in [um] (default is 250 um).
--tm-stim-location {endo,epi}
choose transmural stimulus location (default is endo)
--ab-stim-location {apex,mid,base}
choose apicobasal stimulus location (default is mid)
--bath BATH
choose size of bath to attach at epi and endo [mm]
(default is 0.0 mm). With increasing the bath size
the difference between recovered extracellular potentials
and (pseudo-)bidomain potentials diminishes.
--sourceModel {monodomain,bidomain,pseudo_bidomain}
pick type of electrical source model (default is monodomain).
For comparing, pseudo_bidomain is most appropriate
as compute time with bidomain is markedly longer.Expected Results
In the monodomain case where we do not compute potentials in the bath domain, only recovered extracellular potentials
can be inspected. In fig-tutorial-ecg-monodomain expected results are illustrated.
Recovered extracellular potentials and corresponding field points are shown.
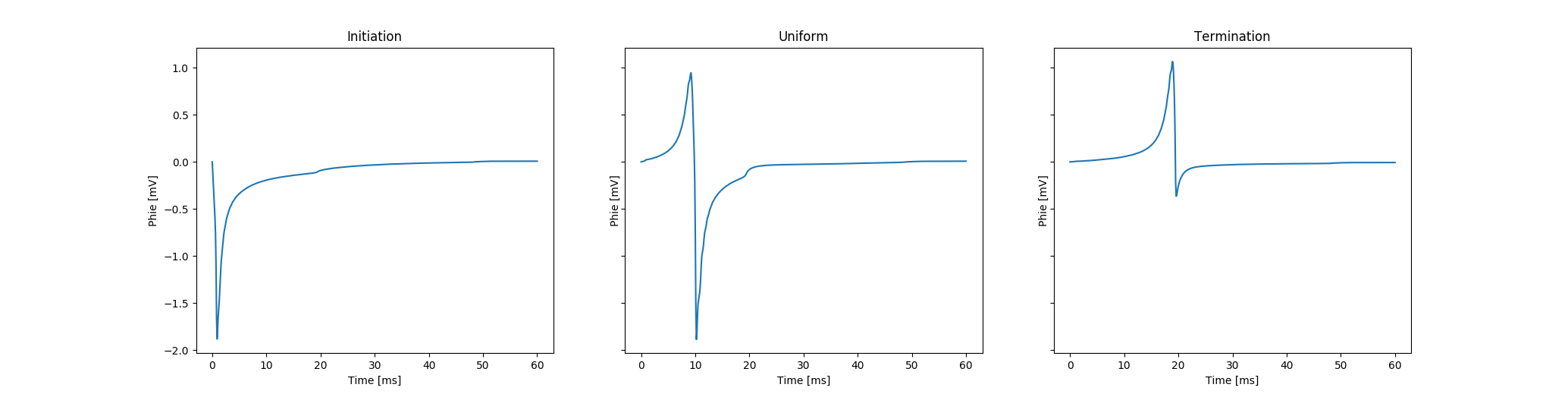
fig-tutorial-ecg B),
uniform undisturbed propagation (\(\phi_{\rm 1}\) in fig-tutorial-ecg B)
and terminating (or colliding) propagation (\(\phi_{\rm 2}\) in fig-tutorial-ecg B).Activation spread in terms of transmembrane voltage \(V_{\rm m}\) and extracellular potential \(\phi_{\rm e}\)
are shown in fig-tutorial-ecg-excitation-spread.
Experiments
Experiment exp01 (central endocardial activation (monodomain) with ecg recovery)
In a first step we omit a bath and use a monodomain as a source model. Extracellular potentials are computed using the \(\phi_{\rm e}\) recovery technique. Propagation is initiated at the center of the endocardial surface as if activated by a Purkinje fascicle. Only 60 ms of activation are computed as we are only interested in the depolarization complex. Details on the openCARP-specific definition of this method are found in the manual.
./run.py --sourceModel monodomain --duration 60 --visualizeIn this case, the extracellular potentials at the sites of stimulus delivery (vertex 0, initiating propagation), in the mid-myocardium (vertex 1, undisturbed uniform propagation) and at the epicardial surface (vertex 2, terminating propagation) shall be examined.
Experiment exp02 (apical endocardial activation (monodomain) with ecg recovery)
In experiment exp02 we shift the stimulus towards the apical edge of the wedge at the endocardium. The observation sites for recovering \(\phi_{\rm e}\) remain the same, but local activation patterns underneath the recording electrodes are changed. This is reflected in the waveforms of the unipolar extracellular electrograms. Total activation time in this case is longer, which is why we pick a larger duration to allow for the entire wedge being activated.
./run.py --ab-stim-location apex --sourceModel monodomain --duration 100 --visualizeExperiment exp03 (apical endocardial activation (pseudo-bidomain) with ecg recovery, small bath)
We repeat exp02 using a pseudo-bidomain source model. This allows the comparison between extracellular potentials computed by the bidomain method with those computed by the recovery method. We start with a very small bath size of only 1 mm.
./run.py --ab-stim-location apex --sourceModel pseudo_bidomain --bath 1.0 --duration 60 --visualizeExperiment exp04 (apical endocardial activation (pseudo-bidomain) with ecg recovery, larger bath)
We repeat exp03 using a larger bath of 20 mm width to examine how bath size effects extracellular potential waveforms, their magnitude and differences between bidomain and recovery potentials.
./run.py --ab-stim-location apex --sourceModel pseudo_bidomain --bath 20.0 --duration 60 --visualizeExperiment exp05 (apical endocardial activation (monodomain) with ecg recovery, repolarization)
We repeat exp02, but run for longer to observe unipolar electrograms during repolarization.
./run.py --ab-stim-location apex --sourceModel monodomain --duration 400 --visualizeIn this case it is of interest to examine so-called transmural ECGs, that is, the difference between unipolar electrograms recorded from endocardial and epicardial surfaces. The recovered unipolar ECG traces are stored in the file phie_recovery.igb in igb format in the respective output directories. We use igbextract to compute the differences from the igb file and store the output in a text file for easier visualization. For this end we run:
./run.py --tmECG simIDwhere simID is the name of the output directory for which we want to compute the transmural ECG.
The computed transmural ECG can be visualized, for instance, with gnuplot.
References
Bishop MJ, Plank G., Representing cardiac bidomain bath-loading effects by an augmented monodomain approach: application to complex ventricular models., IEEE Trans Biomed Eng 58(4):1066-75, 2011. [Pubmed] [PMC]↩︎
Bishop MJ, Plank G. Bidomain ECG simulations using an augmented monodomain model for the cardiac source. IEEE Trans Biomed Eng 58(8):2297-2307, 2011. [Pubmed] [PMC]↩︎
Boukens B, Sulkin MS, Gloschat CR, Ng FS, Vigmond EJ, Efimov IR. Transmural APD gradient synchronizes repolarization in the human left ventricular wall. Cardiovasc Res 108(1):188-96, 2015. [Pubmed] [PMC]↩︎
Recent questions tagged experiments, examples, tissue, ecg, monodomain, bidomain, pseudo-bidomain
There are tagged with experiments, examples, tissue, ecg, monodomain, bidomain, pseudo-bidomain.
Here we display the 5 most recent questions. You can click on each tag to show all questions for this tag.
You can also ask a new question.
